What Exactly Is Rubber?
The Structure of Rubber and Self-Healing Rubber
NOK's flagship product, the oil seal, is mainly made of rubber. The reason NOK has been able to endow oil seals with such a wide range of functionalities to meet society's needs is its long-standing dedication to original research on rubber. But what exactly is rubber? This article delves into NOK's investigations into the nature of rubber.
What is rubber?
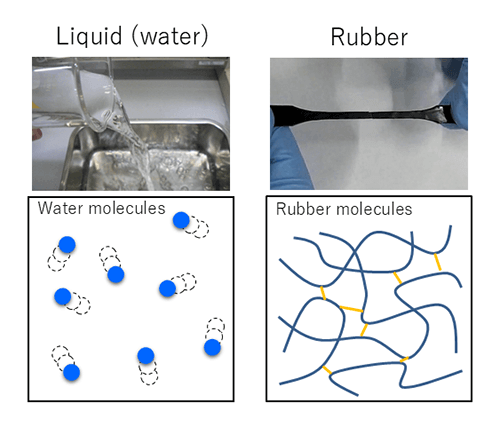
So, what exactly is rubber? According to Yuichi Aoyagi from the Material Research Section of NOK R&D's Engineering Research Department, rubber is a material that "looks like a solid but is closer to a liquid." Rubber has the unique ability to stretch and contract in response to external forces and then return to its original shape once the force is removed (elasticity). While this property of rubber is something everyone is familiar with, it is deeply rooted in rubber's microscopic structure.
Let's consider liquids. If you magnify a liquid and take a look, you will see countless molecules moving freely. Rubber is similar to a liquid, since it is also made up of numerous moving molecules. However, unlike water and other liquids, rubber consists of intricately entangled chain-like molecules. Moreover, these molecules are connected by bridge-like structures known as "cross-links," which restrict the movement of the molecules. This is why water flows and spills easily with its free-moving molecules, while rubber retains its shape and does not flow like a liquid.
Please enlarge the screen to view
Rubber supports society
Rubber is an indispensable material in modern life. One reason is its elasticity, which allows it to control the flow of liquids. A prime example is the packing used in water faucets. When you turn a faucet, the rubber packing inside creates a seal that blocks the water. Turning the faucet loosens this seal, creating a gap that allows the water to flow through. Turning the faucet creates a gap that allows the water to flow through. Many machines and products around us rely on rubber's sealing functionality.
However, understanding and handling those properties is crucial to avoid serious accidents. The Space Shuttle Challenger explosion in January 1986 was caused by a failure in the sealing O-ring of the solid rocket booster, which allowed fuel to leak through the gap between the O-ring and the shuttle. Though only a few millimeters thick, the O-ring had a diameter of 3.65 meters. The freezing temperatures on the launch day caused the rubber's elasticity to diminish, leading to the leakage. At NOK, a company that manufactures O-rings, such incidents highlight the importance of fundamental research into rubber to prevent such accidents.
NOK's rubber research
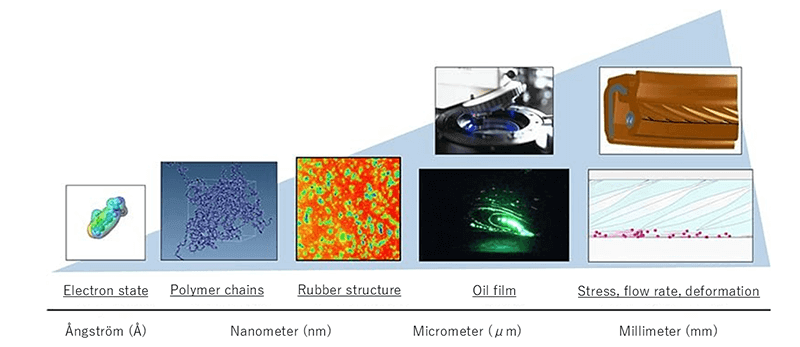
The history of rubber is long, but much about its microscopic structure and properties remains unknown. Aoyagi notes: "At NOK, we prioritize visualizing and controlling the microscopic structure of materials." This research spans a wide range of scales, from nanoscale (billionths of a meter) dispersion structures to microscale (millionths of a meter) oil films and millimeter-scale product stresses and deformations. This broad-spectrum (multi-scale) research enables the development of features and properties that meet user needs and leads to the creation of entirely new functions.
Please enlarge the screen to view
Innovative rubber products for a sustainable society
Rubber's properties are closely tied to its cross-linked structure. Traditionally, rubber was expected to withstand tension, pressure and harsh environmental conditions like high temperatures and ultraviolet radiation. To achieve these qualities, a process known as vulcanization*1 was essential to introduce cross-linked structures and enhance elasticity.
Recently, as products contributing to reduced environmental impact and a more sustainable society have gained attention, rubber has also been called on to offer new functions. NOK has focused on ionic bonds*2 as a type of chemical bonding and developed "Links Rubber", which uses ionic bonds to fulfill the role of cross-links. "Links Rubber" has "self-healing" properties, enabling products to be recycled as-is. By reshaping and reusing end-of-life products, NOK seeks to create a circular economy in industries like automotive manufacturing. "We hope to make "Links Rubber" a representative material of the sustainable society everyone dreams of," says Keita Suzuki from the Technical Research Department, Materials Research Section.
Please enlarge the screen to view
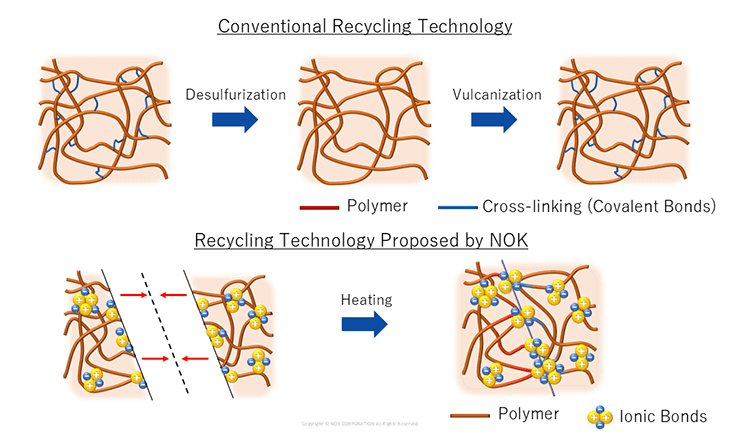
- The process of increasing rubber elasticity by creating cross-linking structures. This is done by kneading sulfur powder into the polymer, which serves as the raw material for rubber, and then heating it at high temperatures.
- A chemical bond resulting from the electrostatic attraction (Coulomb force) between cations and anions. Unlike conventional rubber, "Links Rubber" forms cross-linking structures through ionic bonding. This enables the rubber to rebind when pressure is applied to cut sections, a property referred to as self-healing.
(Photo left)
Yuichi Aoyagi
Materials Research Section, R&D Technical Research Department, Materials Research Section, NOK Corporation
Yuichi Aoyagi specialized in quantum chemistry during his graduate studies. After joining NOK, he worked on rubber material research and the development of analytical techniques. He was seconded to NOK's partner company, Freudenberg (Germany), where he researched the mechanisms of seal rubber materials degradation at the DIK (German Institute for Rubber Technology) in 2018 and earned a doctorate. He is currently involved in material research activities supporting the foundation of the NOK Group.
(Photo right)
Keita Suzuki
Materials Research Section, R&D Technical Research Department, NOK Corporaration
Keita Suzuki specialized in organic and inorganic chemistry during his graduate studies. After joining NOK, he was assigned to the Shonan R&D Center. He works in the Engineering Research Department's Materials Research Section, focusing on research into rubber cross-linking mechanisms and the control of cross-linking structures. He is also involved in developing new materials, such as "Links Rubber", and advancing analytical technologies.
- Note: Data, positions and titles in this article are current as of July 2023.