Preventing Troubles Before They Happen: The Struggle to Uncover the Mechanisms Behind Material Degradation
Automobiles, electronic products, information and communication devices, air-conditioning systems, vending machines, manufacturing equipment installed in factories, and the other machinery surrounding us are not immune to failure. While these occurrences are not frequent enough to be considered common, most people have experienced an incident caused by a mechanical failure or heard about one happening nearby.
The root cause of mechanical troubles is often found in the machinery's components. Even the slightest misalignment or barely visible cracks in a small part can lead to malfunctions, potentially resulting in accidents that put human lives at risk.
Understanding the mechanisms of material degradation is vital when investigating the nature of defects and the accidents they cause, and when designing components that prevent such incidents. In this context, the following sections explain the mechanisms behind rubber degradation based on NOK's extensive research over the years.
Rubber, with its unique ability to stretch and contract under external forces and return to its original shape when the force is removed, is widely used in various applications. Often functioning in unseen but crucial roles, rubber is an essential component supporting the operation of many devices.
Understanding degradation mechanisms to prevent accidents
Past incidents demonstrate the importance of using materials and components appropriately, including rubber, and maintaining optimal operating environments. For example, the catastrophic explosion of the Space Shuttle Challenger on January 28, 1986, has been attributed to a rubber component failure. On a smaller scale, carbon monoxide poisoning incidents linked to defective kerosene fan heaters occurred in Japan around 2005. Despite the differences in circumstances and specific parts involved, both cases have been reported as stemming from issues with rubber components.
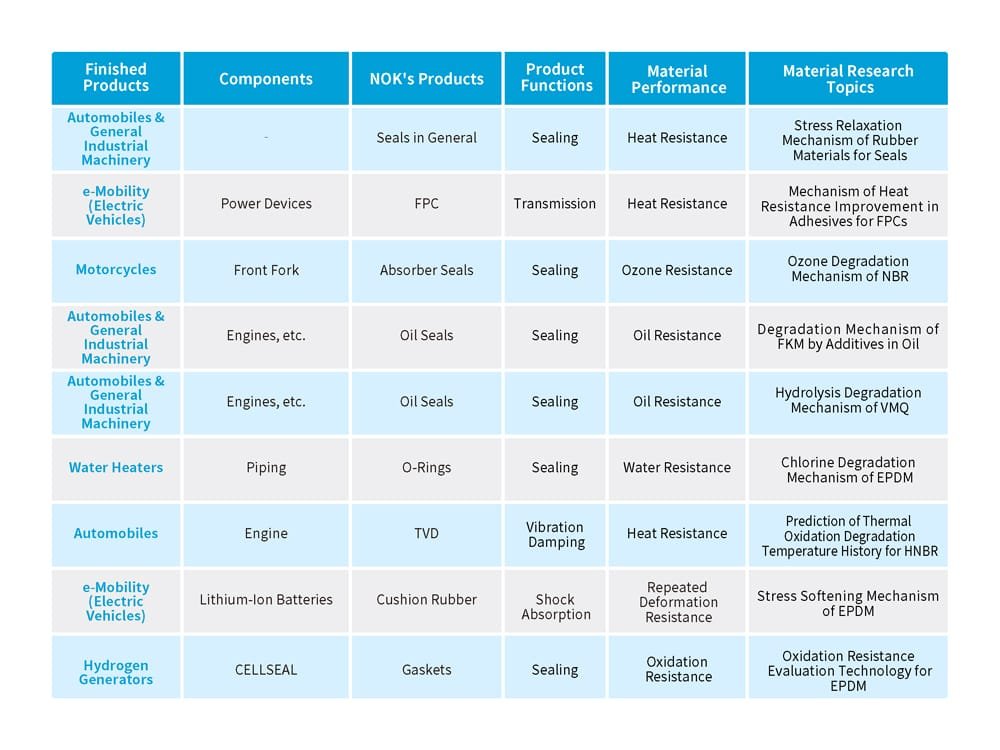
Rubber degrades due to various factors. Observable changes include cracking from repeated stress and wear from abrasion. In other cases, the material may lose its original hardness or softness. Its elasticity — its ability to return to its original shape — may also deteriorate. Table 1 provides an overview of some research themes that NOK is pursuing on rubber degradation.
Please enlarge the screen to view
This section highlights examples of the degradation mechanisms of rubber materials used in seal products for automobiles and other applications. As part of its automotive product lineup, NOK uses rubber materials in oil seals — which prevent oil leaks from rotating shafts in engines and geared motors — and gaskets that prevent hydrogen gas leaks in fuel cells (Figure 1).
Please enlarge the screen to view

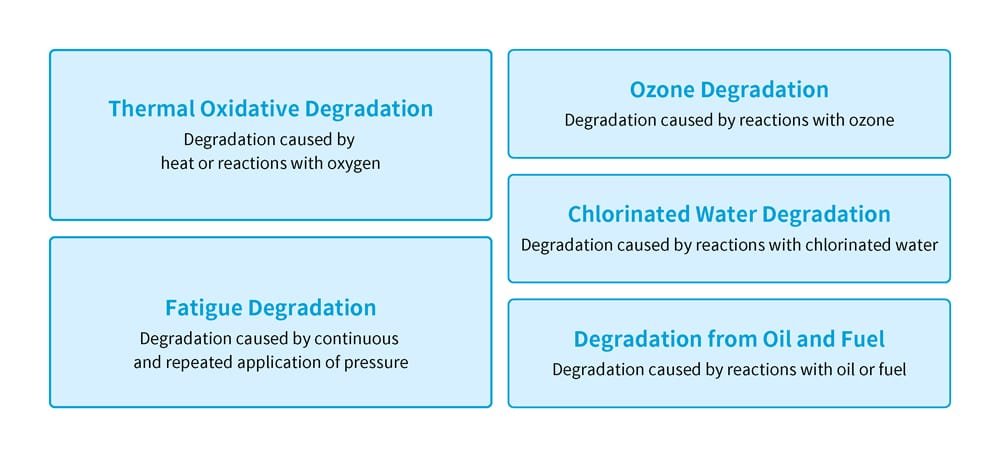
The factors causing rubber degradation can be categorized into several patterns (Figure 2). These include thermal oxidative degradation, where the material deteriorates due to heat or a reaction with oxygen; fatigue degradation, caused by continuous and repeated pressure; and degradation due to the influence of chemical substances in the environment, such as ozone, chlorinated water, oil or fuel. These degradation phenomena often involve a combination of physical changes and chemical changes resulting from reactions with certain substances.
Please enlarge the screen to view
First, let's examine the stress relaxation mechanism, represented by a permanent compression set — a hallmark of thermal oxidative degradation, where the material fails to return to its original shape after deformation.
Stress relaxation in rubber: Combining elastic and viscous properties
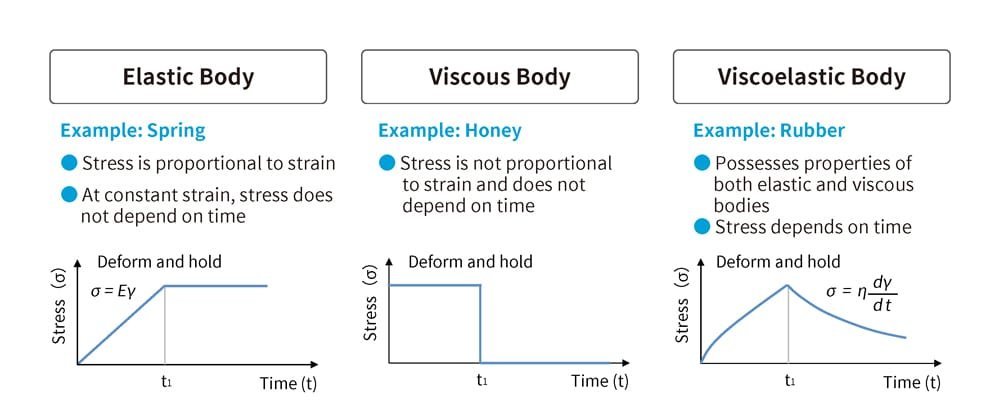
When an external force (external load) is applied to an object, an internal force (internal stress) arises in response. The magnitude of this internal force is expressed as a physical quantity called "stress." For example, a spring deforms according to the force applied but returns to its original shape when the force is removed. Such materials are called "elastic bodies." On the other hand, materials that resist applied forces like fluids but do not return to their original shape when the force is removed are called "viscous bodies." Rubber possesses characteristics of both elastic and viscous bodies, making it a material known as a "viscoelastic body." While it appears solid, its properties are closer to that of a liquid (Figure 3).
Please enlarge the screen to view
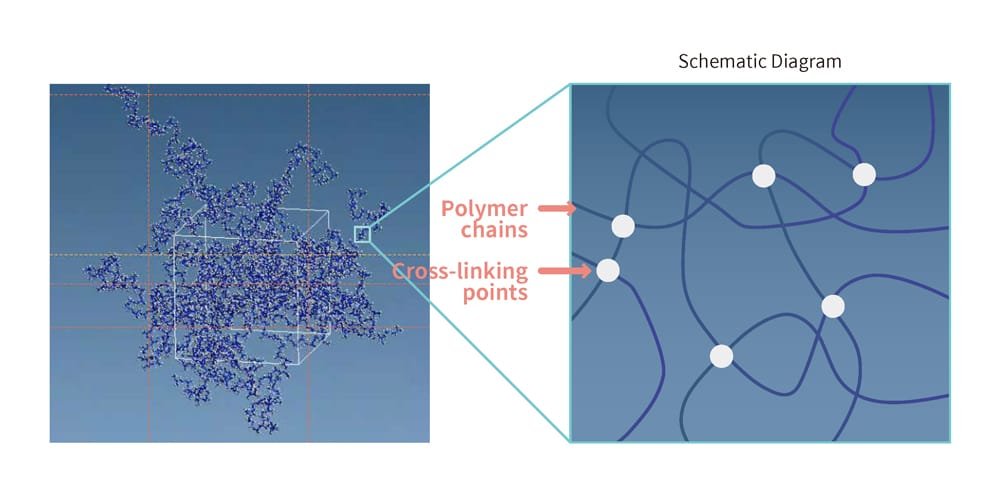
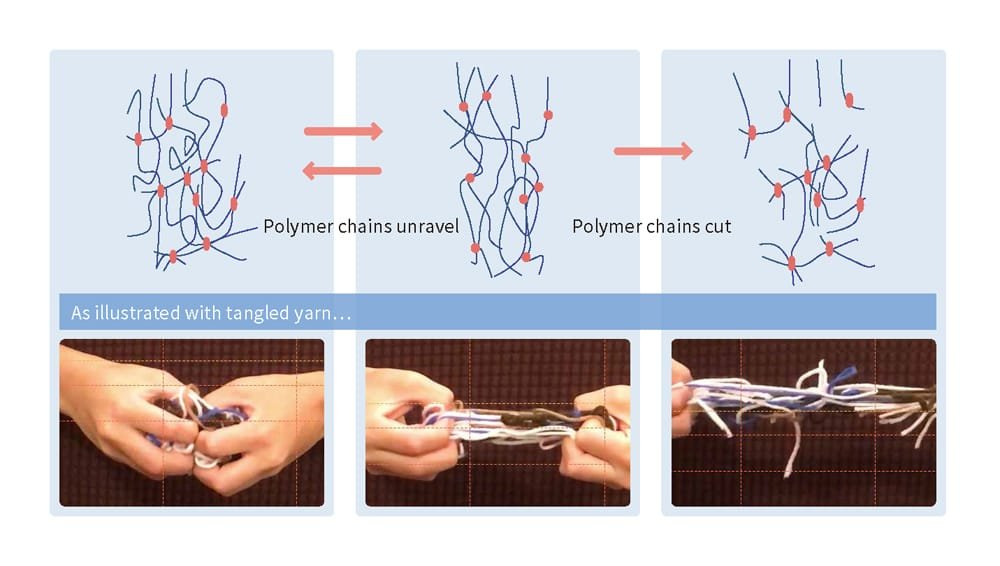
The distinctive properties of rubber — such as its ability to stretch and contract — are rooted in its microscopic structure. Specifically, rubber is composed of countless moving molecules, similar to those in liquids. Unlike liquids,however, the chain-like polymers in rubber are intricately entangled, with additional cross-linkages connecting these polymers at certain points. This is referred to as a cross-linked structure, and it prevents the polymers from separating by restricting their movements (Figure 4). This allows the rubber to freely change shape in response to external forces while maintaining the connections between the polymers. Once the external force is removed, the material returns to its original shape. The more cross-links present, the stronger the restrictions on polymer movement, making the material harder to deform. Conversely, fewer cross-links result in reduced restrictions, making it harder for the material to return to its original shape after deformation.
Please enlarge the screen to view
This structure can be likened to a tangle of yarn. When a tightly entangled ball of yarn is pulled from both sides, it resists coming apart and attempts to return to its original state. However, if the yarn is cut in the middle with scissors, it gradually unravels. Rubber is similar: as the cross-links — connections between the polymers — begin to unravel, the stress gradually weakens, and the physical properties of the rubber deteriorate (Figure 5).
Please enlarge the screen to view
Rubber may not display significant changes even as its stress diminishes. In most cases, in fact, the external appearance remains unchanged while the internal cross-linked structure gradually evolves.
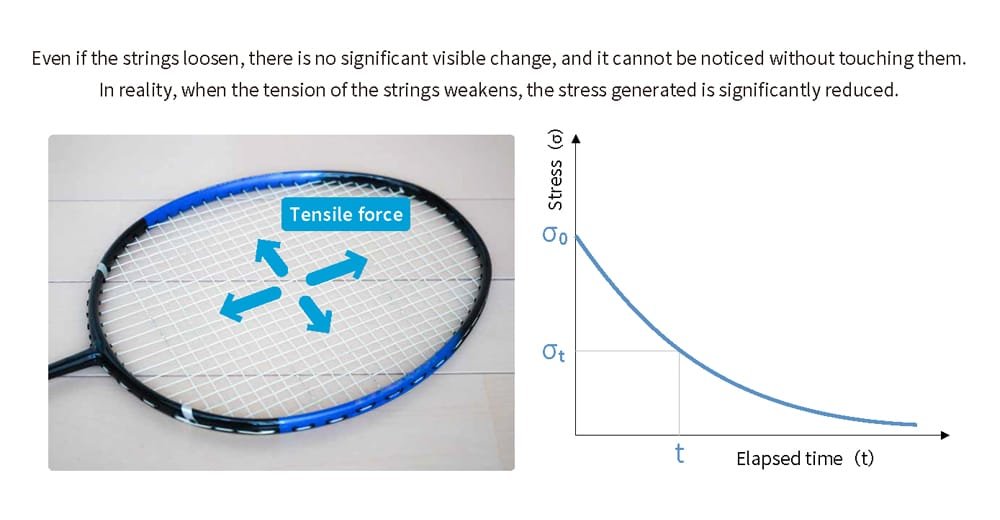
Let's consider a familiar example where stress decreases without visible changes. Imagine the strings of a tennis or badminton racket. With repeated play, as balls or shuttlecocks are struck, external forces gradually loosen the tension (tensile force) in the strings. Even when not in use, the tension of the strings naturally decreases over time if the racket is left unused. Although the strings may look unchanged, when used, it becomes clear that the stress generated when hitting the ball or shuttlecock has significantly diminished (Figure 6).
Please enlarge the screen to view
There are various causes of stress relaxation. In some cases, the entanglement of polymer chains unravels over time due to regular use and aging, while in others, cross-links are severed due to temperature changes or pressure. Chemical reactions with substances like oxygen and ozone are also common causes. Understanding these mechanisms in conjunction with the operating environment makes it possible to predict rubber degradation in advance, and to design materials or products less prone to deterioration. Monitoring the state of rubber through chemical analysis during use can also help prevent failures or accidents. How can we investigate these degradation processes? In the next section, we will introduce methods for examining structural changes in rubber that lead to stress relaxation.