Stretch, Compress, and Return to Form.
NOK Group’s R&D Explores the Unique Material of Rubber
Unlocking the Source of NOK Group’s Value: Rubber
(Part 1)
NOK began as Japan's first oil seal manufacturer, and for over half a century, rubber — the core material behind those seals — has been a central focus of its research. To perform in automotive environments, rubber must withstand oil, endure extreme engine heat, and remain flexible even in freezing temperatures. This article explores how NOK's rubber research has continually evolved to meet the demands of a changing world.
Is rubber a solid or a liquid? A closer look at the molecular level
Rubber bands, gloves, hoses. Rubber is everywhere in our daily lives. But look a little closer, and you'll find this everyday material has properties unlike anything else.
Think about paper: pull it and it tears. Wood dents or cracks under enough pressure. Most materials change shape when force is applied and don't return to their original form. Rubber is different. It stretches, compresses, and springs back once the force is removed. This ability is what we call elasticity.
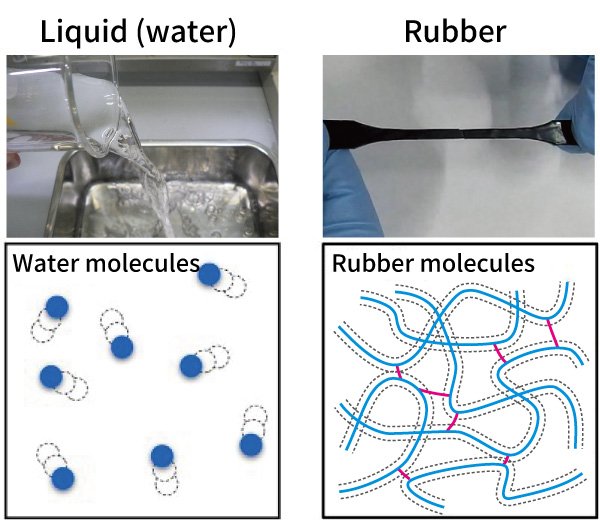
Rubber is highly flexible. And when it comes to the basic states of matter — solid, liquid, or gas — it's often said to resemble a liquid more than a solid. So, what's happening at the molecular level?
Liquids are made up of countless molecules in constant motion. They don't hold a fixed shape but adapt to whatever container they're in. Rubber is also composed of numerous moving molecules but with one key difference: its molecules are long, chain-like structures of many atoms, all tangled together in a dense network. These chains are then tightly bonded by elements like sulfur. That structure is what gives rubber its bounce. Even when stretched, those interlinked molecular chains hold together and pull the material back to its original shape.
Please enlarge the screen to view
Breaking down rubber's behavior from the microscopic to the visible
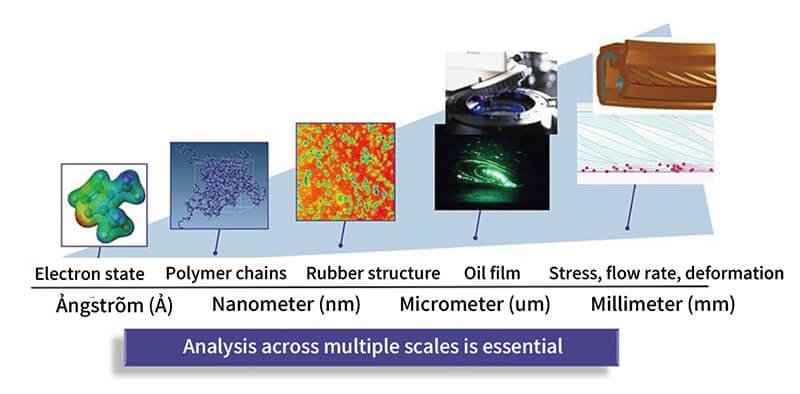
Not all rubber is created equal. Its properties vary depending on the product's shape, the substances mixed into the rubber, and its molecular structure. That's why developing rubber products requires understanding structures across a wide range of scales, from the visible millimeter level down to the nanometer scale, which is one-billionth of a meter and far beyond what the eye can see.
At NOK, researchers explore rubber at every level: from the behavior of electrons measured in angstroms to nanometer-scale structures, micrometer-scale oil films (one-millionth of a meter), and even the stresses acting on finished products at the millimeter scale. This multiscale approach enables broad, ongoing research into the materials and forces at play.
Please enlarge the screen to view
Understanding rubber at the molecular level starts with having the right tools. At NOK's Shonan R&D Center in Fujisawa, over 40 advanced instruments are in use. Among them are technologies originally developed for medical use, like nuclear magnetic resonance for probing molecular structures, as well as atomic force microscopes that can reveal surface textures at the nanoscale, and X-ray photoelectron spectroscopy to analyze surface composition.
But NOK doesn't work alone. The team regularly collaborates with universities, research institutions, and other companies — including Germany's Freudenberg Group, a long-term partner since 1960 — sharing knowledge and talent to push the boundaries of what's possible in measurement and analysis.
This deep commitment to chemical research is what underpins the reliability and precision of NOK's manufacturing.
Images courtesy of Journagram, "What Exactly Is Rubber? The Structure of Rubber and Self-Healing Rubber"